Services and Rates
Regular Hours: Mon – Fri 8:00 am – 7:00 pm
Discounted Hours: Sat & Sun all day; Mon – Fri 7:00 pm – 8:00 am
There is a $1.30/hour charge for nitrogen gas, which includes any special use of nitrogen including hi T or low T work.
Consultation refers to use of facility staff time for training, assistance in setting up experiments, assistance with processing data, discussions of NMR experiments, etc. Samples run by staff include the consultation rate only for staff time used in setting up the spectrometer and sample and for processing.
Rates are subject to change.
NMR Hourly Rates
| UNC-CH | Off Campus Academic | Commercial/Industry | |
|---|---|---|---|
| Regular Hours | $14 | $48 | $80 |
| Nights & Weekends | $10 | $27 | $80 |
| Helium Cryoprobe Surcharge | $5 | $11 | $15 |
| Nitrogen (Prodigy) Cryoprobe Surcharge | $3 | $6 | $8 |
| EPR | $7 | $41 | $45 |
| Consultation | $50 | $212 | $220 |
NMR Assignment Service
The NMR assignment service is designed to rigorously characterize a molecule for publication and/or intralab use. NMR data will be worked up, assigned, and condensed to a report in publishable format.
The following services are available:
- Connectivity Assignment includes (1H; 13C; 1H-1H COSYDQF; 1H-13C HSQC, HSQC-TOCSY, HMBC) to show bond connectivity of molecules.
- Absolute Assignment (1H-1H NOESY/ROESY): Uses NOE assignment to attempt to assign magnetically inequivalent protons. This can provide an absolute assignment of protons.
- Heteronuclear Assignment (11B, 15N, 19F, 29Si, 31P): Adds experiments to characterize heteronuclear NMR and show heteronuclear connectivity.
- Sample Preparation by NMR Staff is divided into three tiers based on solvent cost
- Tier 1: CDCl3, C6D6, DMSO-d6, D2O, MeOD-d4
- Tier 2: CD2Cl2, toluene-d8
- Tier 3: THF-d8
If this option is selected, staff will prepare your sample with the selected solvent (from freshly opened ampules) for NMR characterization.
NMR Assignment Service Report SOP
Overview
The NMR assignment service is designed to rigorously characterize a molecule for publication and/or intralab use. NMR data will be worked up, assigned, and condensed to a report for publication in the supplemental information (SI) files or electronic notebooks.
The following services are available:
- Connectivity Assignment includes (1H; 13C; 1H-1H COSYDQF; 1H-13C HSQC, HSQC-TOCSY, HMBC) to show bond connectivity of molecules.
- COSYDQFM:a double quantum filtered 1H-1H COSY, this experiment shows the same information as a default COSY but suppresses singlets to boost data resolution.
- HMBCGP: a 1H-13C HMBC shows long distance proton carbon correlations; a very useful experiment for quaternary carbon assignment when there are nearby protons.
- HSQCEDETGPISP (HSQC-ME): a 1H-13C HSQC-multiplicity edited (HSQC-ME) shows one bond proton-carbon coupling, but also has the advantage of showing how many protons are bound to a carbon. Carbons with 1 or 3 protons will be phased opposite of those with 2, allowing easier assignment of carbon shifts. An excellent replacement for a DEPT135 experiment.
- HSQCETGPML (HSQC-TOCSY): a 1H-13C HSQC-TOCSY provides correlation maps of all protons and carbons coupled in a spin system. While technically this information could be gathered using HSQC’s and COSY correlations, the map that an HSQC-TOCSY provides make assignment of spin systems much easier, since all of the data is in the same place.
- Absolute Assignment (1H-1H NOESY/ROESY): Uses NOE (Nuclear Overhauser Effect) correlations to attempt to assign magnetically inequivalent protons. This can provide an absolute assignment of protons.
- NOESYPHSW: a 1H-1H NOESY provides through space coupling information rather than through bond. Cross peaks show that protons are spatially proximal, even when far apart on the molecule.
- ROESYPHSW: a 1H-1H ROESY provides through space coupling information rather than through bond, just like those observed in a 1H-1H NOESY; this experiment can have enhanced through space correlation sensitivity for compounds with a weak NOE – compounds with approximately MW 700-1200.
- Heteronuclear Assignment (11B, 15N, 19F, 29Si, 31P): Adds experiments to characterize heteronuclear NMR and show heteronuclear connectivity.
- Sample Preparation by NMR Staff is divided into three tiers based on solvent cost
- Tier 1: CDCl3, C6D6, DMSO-d6, D2O, MeOD-d4
- Tier 2: CD2Cl2, toluene-d8
- Tier 3: THF-d8
If this option is select, staff will prepare your sample with the selected solvent (from freshly opened ampules) for NMR characterization.
Accessing Assignment Service Request
All assignment service requests are handled under the “Request Services” Tab in iLab. Choose “initiate request” button next to the “NMR Molecular Assignment tab to access the submission form.
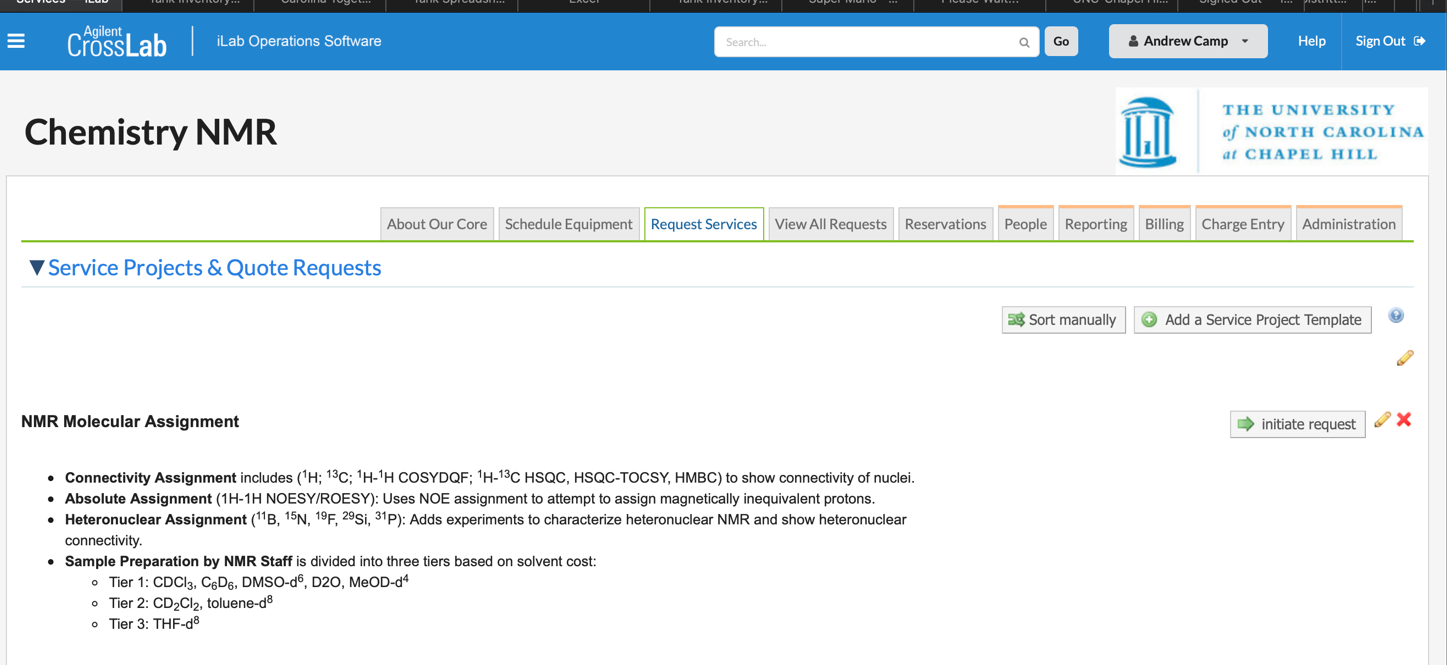
Once the form is opened, fill in the requested information for the sample submission. This includes your onyen, PI, sample name, a picture of the proposed structure(s), and solvent, safety, and stability information. Please ensure that the label in the request form matches that on the vial or tube provided to NMR staff

After all of the relevant sample information is request, please select which services are desired. Type a “1” into the box next to each requested service to add it to the quote. If multiple samples are requested, generate a new form and fill in all of the relevant information for the new sample. Contact NMR staff if a large number of samples are requested to be assigned.
After selecting the desired services, click “Add selected services” once. After a delay, a quote should pop up with the cost of the assignment service. The cost will depend on the whether you are a UNC affiliated or external client. Now click “save completed form” in the next section to save your information.
After submission, NMR staff will contact you with any further questions as well as arrange a time to drop-off your sample.
NMR experiments will be run as soon as possible during the overnight time slots. Additionally, staff may run further experiments if more information is required to assign the molecule.
Report Generation and Assignment
After experiments are run, a summary of the results of the experiments will be provided. This includes publication ready preparation data and worked up spectra for SI use, an assessment of structure validity, and a list of chemical shifts in ACS format. An example report is provided below with connectivity and heteronuclear assignment requested:
10 mg of amc-V-178 was prepared in 500 uL of CDCl3. Samples were run at 25 oC on a Avance III Bruker system equipped with a helium cooled QCI cryoprobe. Spectra were processed with the Mestrenova processing software suite, and baseline correction with the “splines” baseline correction algorithm. Referencing of proton and carbon spectra was done using the residual solvent peak, and fluorine spectra were referenced using absolute referencing.1,2 The NMR features of the sample were found to match the proposed structure (Figure 1).
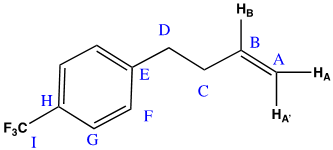
1H NMR (600 MHz, CDCl3) δ 7.55 (d, J = 7.9 Hz, 2H), 7.31 (d, J = 8.0 Hz, 2H), 5.85 (ddt, J = 16.9, 10.2, 6.6 Hz, 1H), 5.06 (ddt, J = 17.1, 1.7, 1.4 Hz, 1H), 5.02 (ddt, J = 10.2, 1.4, 1.4 Hz, 1H), 2.79 (d, J = 7.9 Hz, 2H), 2.41 (tdt, J = 7.8, 6.6, 1.4 Hz, 2H). 13C NMR (151 MHz, CDCl3) δ 145.89, 137.35, 128.74, 128.19 (q, JCF = 32.3 Hz), 125.20 (q, JCF = 3.8 Hz), 124.30 (q, JCF = 270.0 Hz), 115.46, 35.13, 35.08. 19F NMR (565 MHz, CDCl3) δ -62.32.
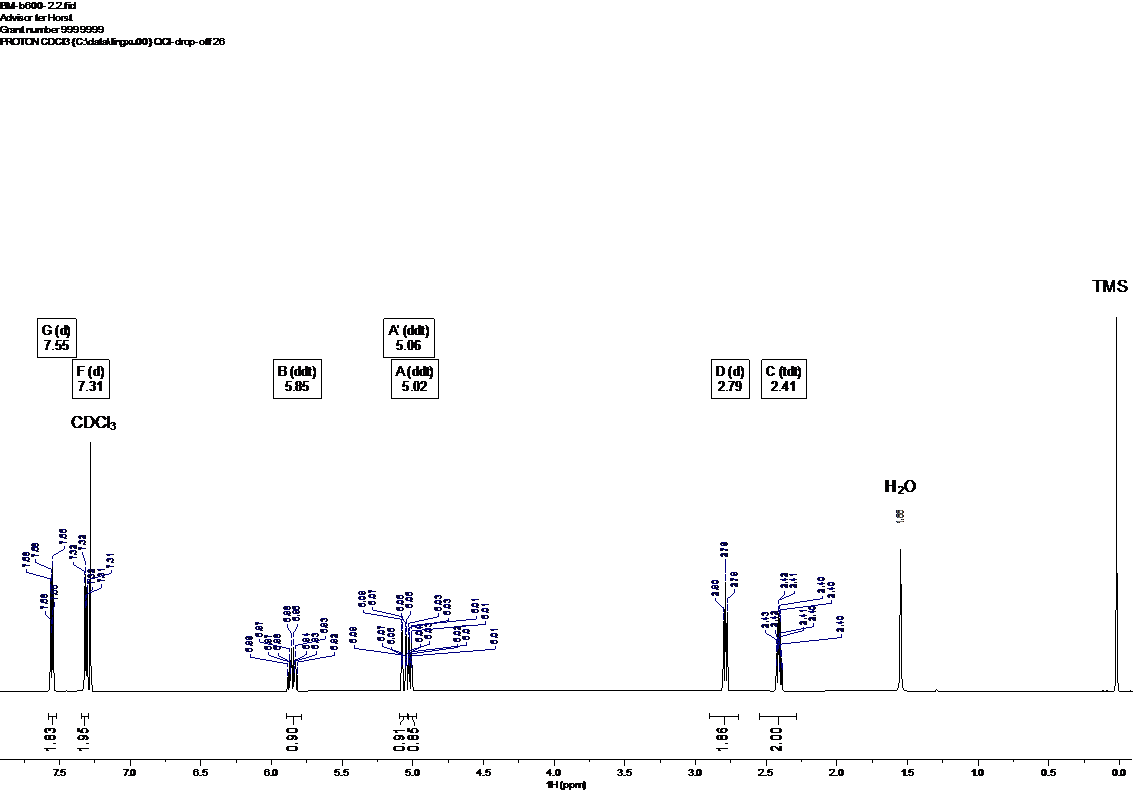
Figure 2. 1H (600 MHz) spectrum in CDCl3

Figure 3. 13C (151 MHz) spectrum in CDCl3
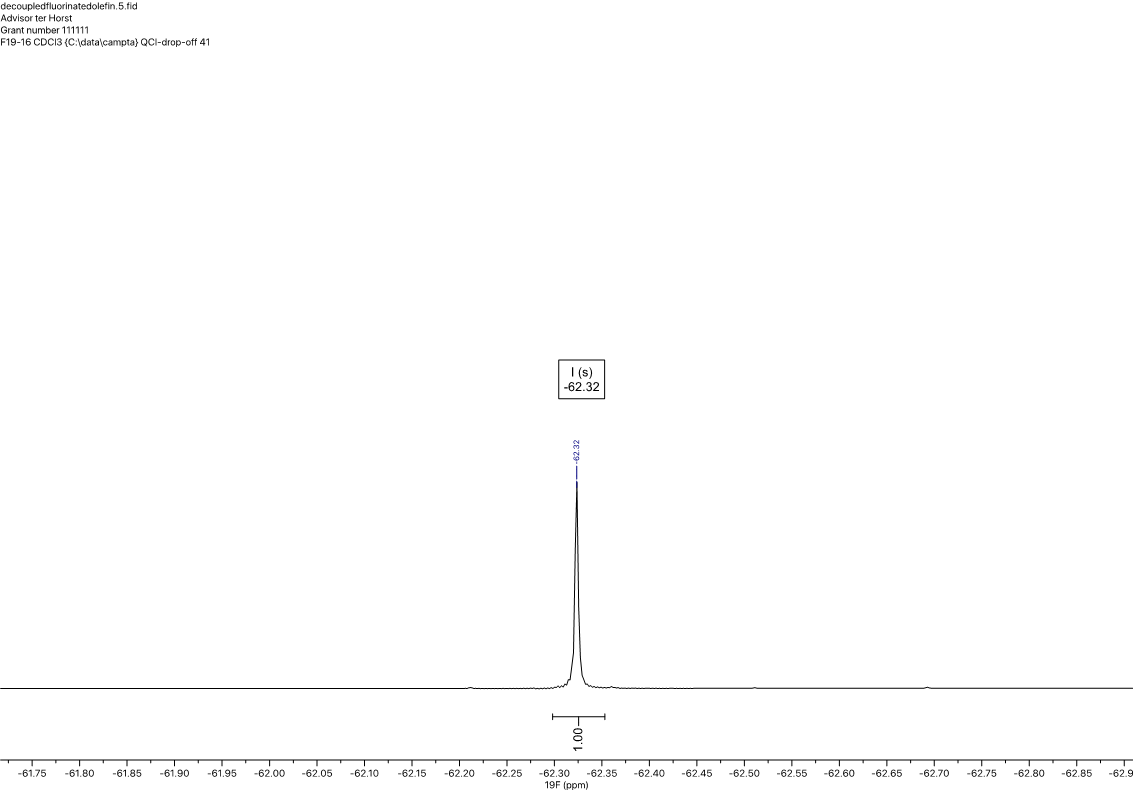
Figure 4. 19F (565 MHz) spectrum in CDCl3

Figure 5. 1H-1H COSY (600 MHz) in CDCl3.
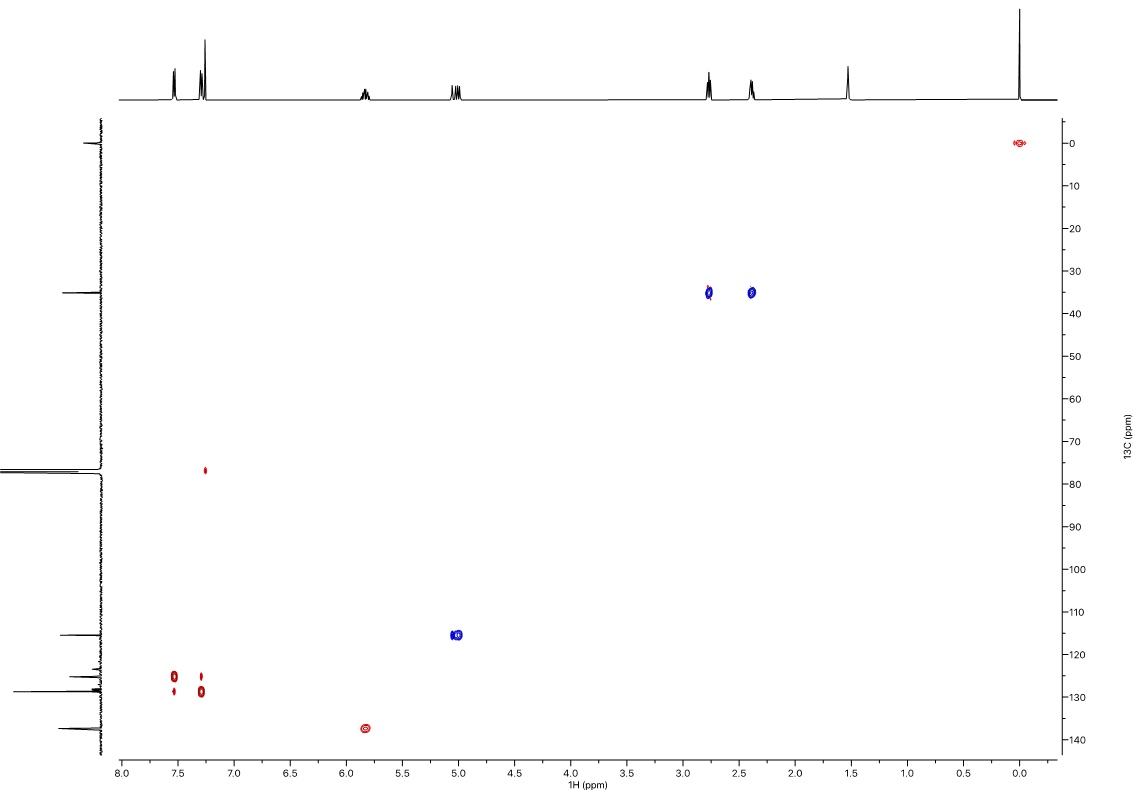
Figure 6. 1H-13C Multiplicity-Edited HSQC (600 MHz) in CDCl3. CH2’s are phased negative (blue), CH’s and CH3’s are phased positive (red).

Figure 7. 1H-13C HMBC (600 MHz) in CDCl3.
Works Cited:
- Harris, R. K.; Becker, E. D.; Cabral De Menezes, S. M.; Goodfellow, R.; Granger, P. NMR Nomenclature: Nuclear Spin Properties and Conventions for Chemical Shifts (IUPAC Recommendations 2001). Concepts Magn. Reson. Part A Bridg. Educ. Res. 2002, 14, 326–346.
- Harris, R. K.; Becker, E. D.; De Cabral Menezes, S. M.; Granger, P.; Hoffman, R. E.; Zilm, K. W. Further Conventions for NMR Shielding and Chemical Shifts (IUPAC Recommendations 2008). Reson. Chem. 2008, 46, 582–598.