Research in the laboratory is centered on understanding the role of structural dynamics in protein function through the use of NMR spectroscopy. In past decades, proteins were essentially viewed as static structures. Today, they are widely appreciated to be dynamic ensembles of interconverting structures. Such behavior can be clearly seen in proteins that undergo dramatic shape changes in different functional states. However, the effects of dynamics can also be important when the structural changes are less apparent. The ensemble nature of proteins has far-reaching implications for understanding basic natural protein functions such as ligand binding, enzyme catalysis, and allostery. An understanding of protein dynamics should lead to improvements in protein engineering and rational drug design.
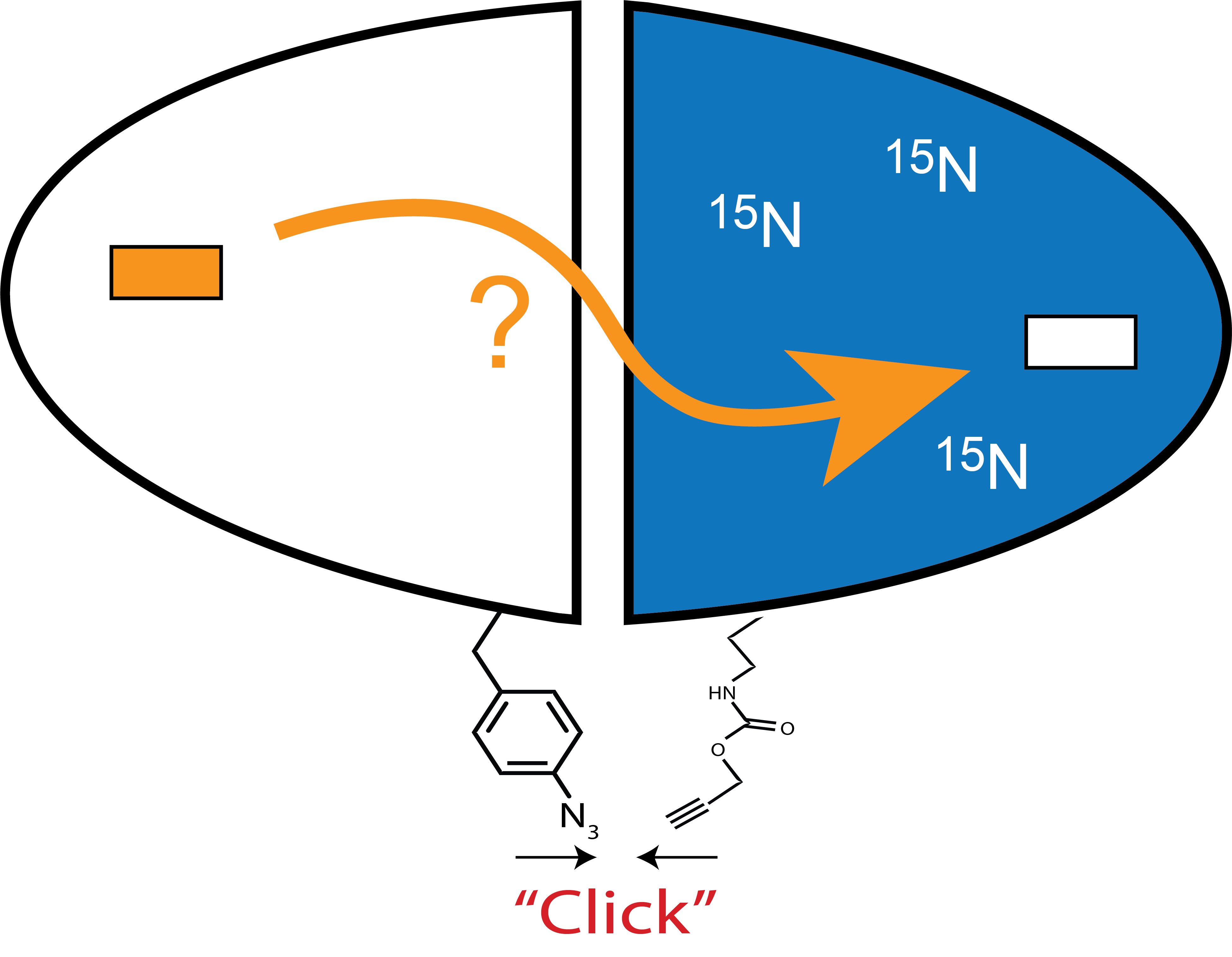
Click Chemistry in Classically Allosteric Proteins
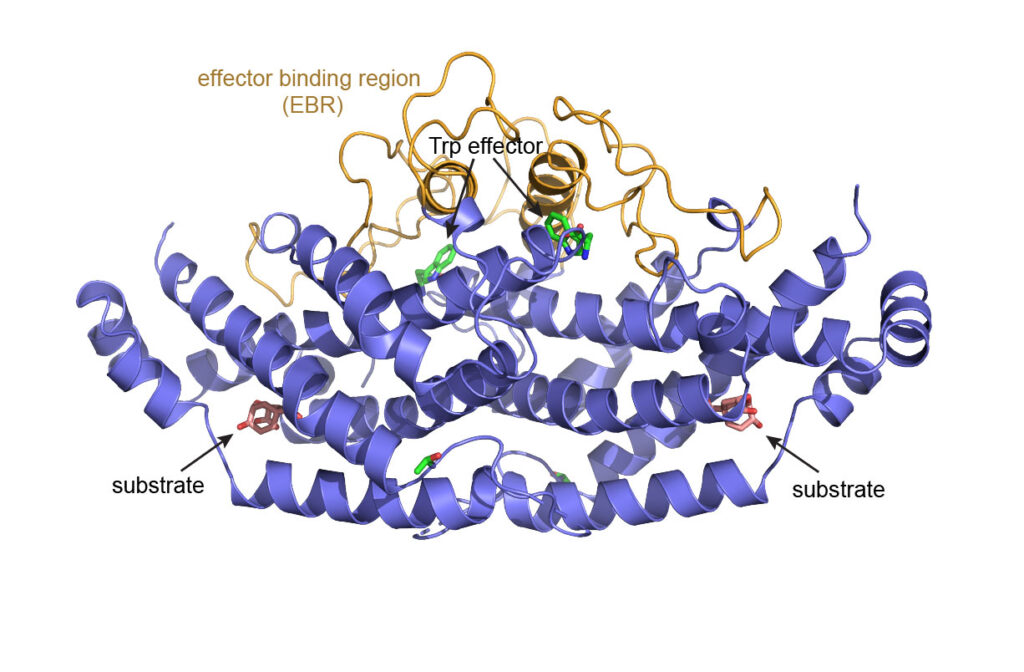
Allosteric Mechanism in Chorismate Mutase
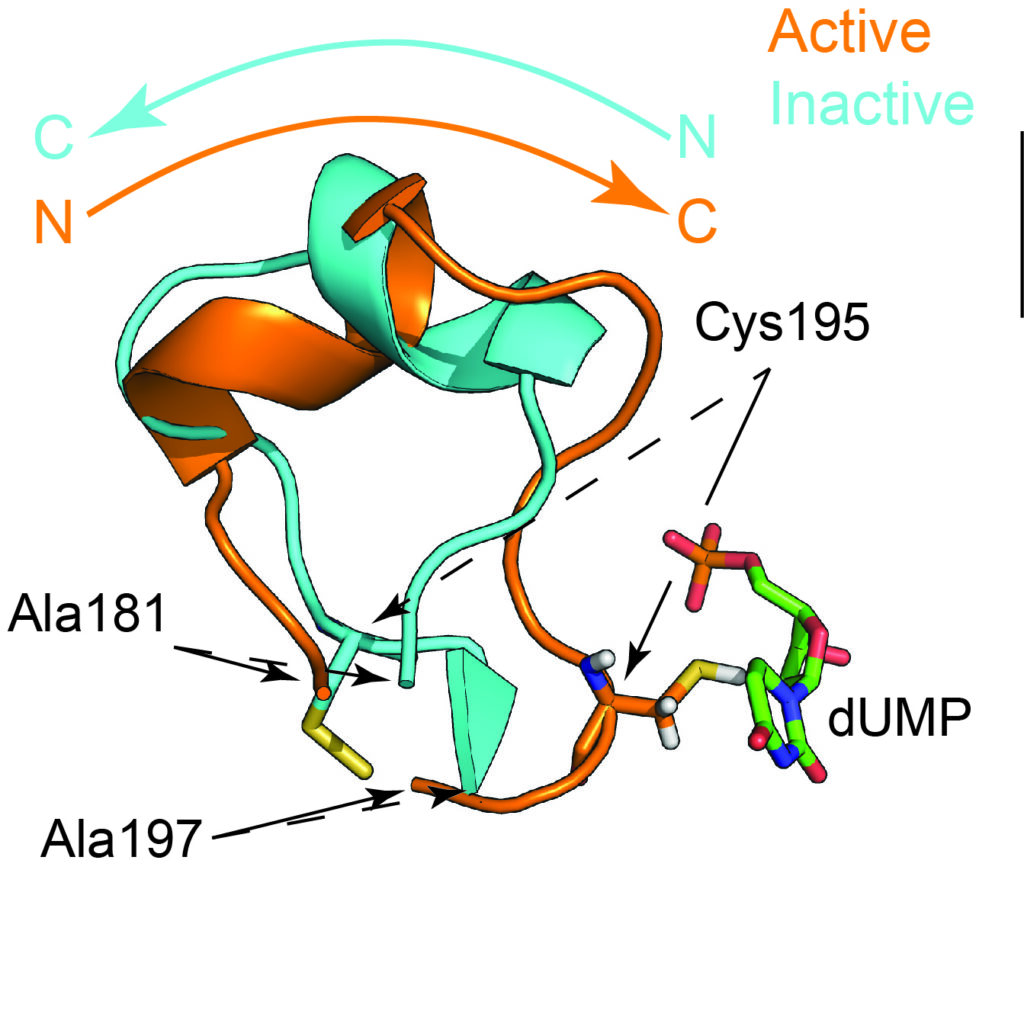
Dynamic Allostery in Thymidylate Synthase
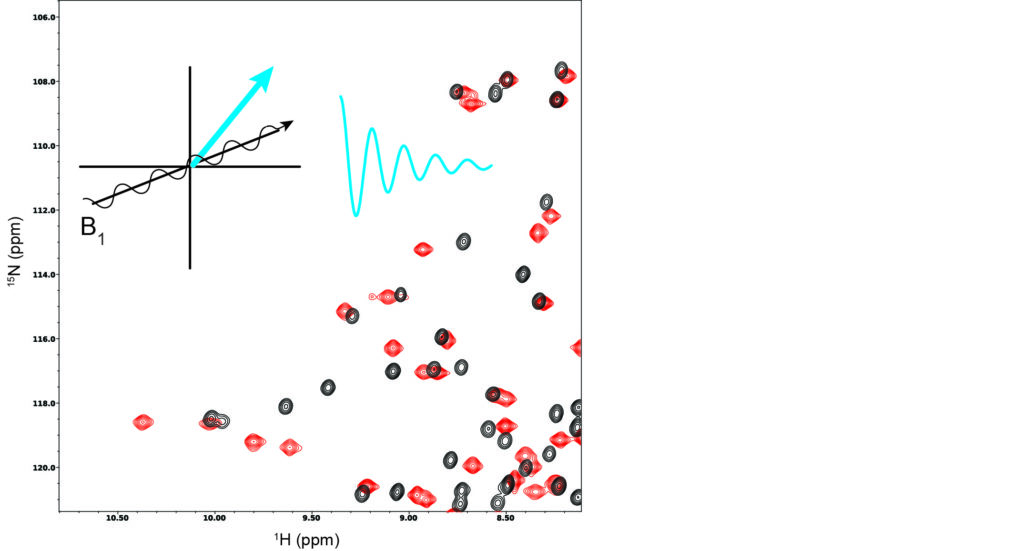
NMR Spectroscopy