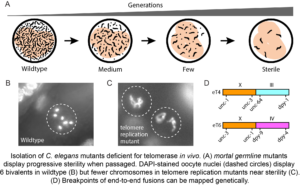
Telomeres are simple repetitive sequences at the ends of chromosomes. In most organisms, telomeres are maintained by telomerase, a reverse transcriptase that adds repeats to chromosome termini. Defects in telomerase have been shown to cause the lethal human hereditary disorders Aplastic Anemia and Pulmonary Fibrosis. Telomerase is deficient in most normal human somatic cells, yet is reactivated in most tumors. When telomeres malfunction in cells deficient for telomerase, they can trigger genome rearrangements, some of which may drive tumorigenesis. We are investigating telomere-induced genome instability and the mechanism by which telomerase adds telomere repeats to chromosome ends.
Telomeres of stressed humans shorten rapidly and this phenotype can be transmitted from mother to child. However, the epigenetic cause of rapid telomere shortening in stressed humans is not understood. We recently identified a form of transgenerational epigenetic inheritance that occurs at nematode telomeres, where germ cells can trigger high or low levels of nuclear telomeric foci for multiple generations. We hope to create models for how stress modifies telomeres and fitness in humans, which may be relevant to a significant segment of society.