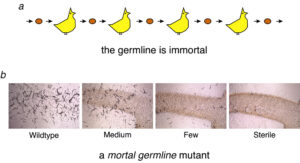
Germ cells are designed to maintain an effectively unlimited proliferative capacity in order to fulfill their biological purpose: to be passed from one generation to the next, indefinitely (Figure 1a). In contrast, somatic cells, including somatic stem cells, are only needed for a single generation. Somatic cells may therefore be deficient for mechanisms that ensure an unlimited proliferative capacity. Biological theory predicts that the soma may have little or no need for processes that allow the germline to remain youthful, vigourous and effectively perfect, for eternity. This dichotomy between germ and soma cells may be the ultimate cause of human proliferative aging and may contribute to age-related diseases such as tumorigenesis. To date, few direct experiments have addressed how germ cells achieve immortality, a fundamental biological process that was first recognized over 100 years ago (A. Weisman, 1892, ‘The Germ Plasm’).
We have isolated C. elegans mutants that are compromised for germ cell immortality. Such mortal germline mutants can reproduce for several healthy generations but then become sterile as a consequence of heritable forms of proliferative damage (Figure 1b). Studies of mortal germline mutants may reveal the mechanisms by which germ cells maintain an unlimited proliferative capacity. This project 1) may provide an understanding how age-related proliferative damage occurs in human stem or progenitor cell populations, and 2) may define forms of heritable damage, aside from genetic mutation, that can be passed from parent to child, thereby influencing human development or disease.