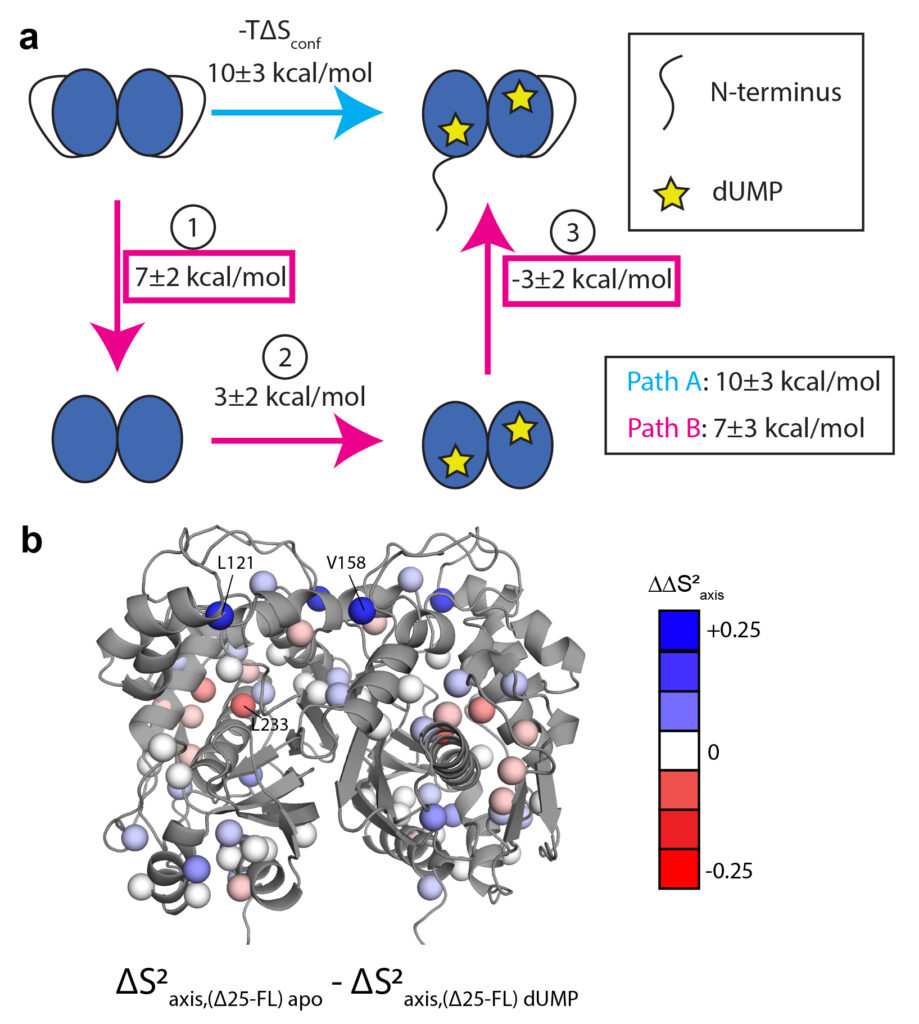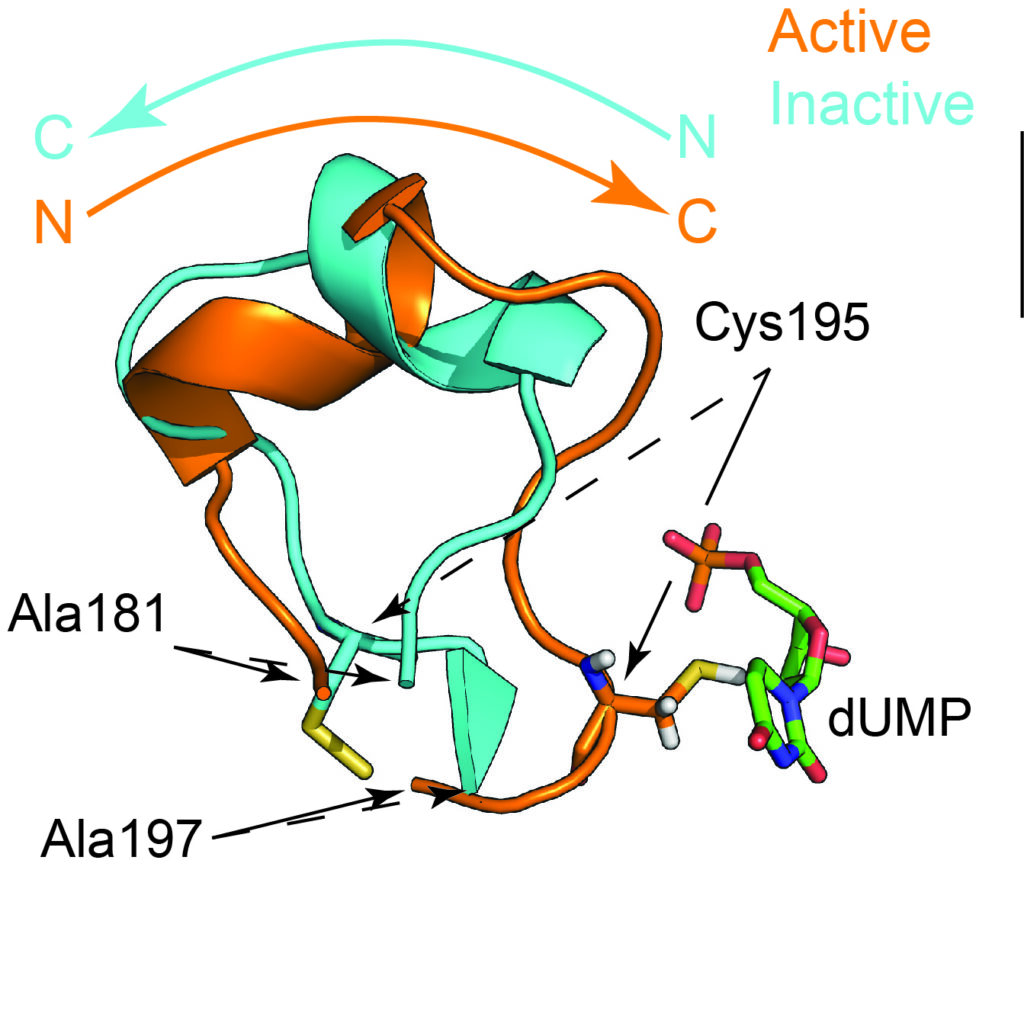
We have been studying the dimeric enzyme thymidylate synthase (or “TS”) for its response to inhibitor/drug binding and its functional allostery. This enzyme is metabolically critical, performing conversion of uridine monophosphate (dUMP) to thymidine monophosphate. Our studies focus on the human and bacterial forms, which show interesting differences in their sequences and substrate binding properties. TS is more complex than many of the enzymes studied to date by NMR, as it has a multi-state reaction coordinate linking substrates and products. This affords an opportunity to track how the dynamics throughout TS change as the enzyme populates different intermediate steps in catalysis. In addition to this interesting reaction mechanism, TS is known to be “half the sites reactive”, meaning that catalytic activity in one subunit imparts non-reactivity to the other subunit. This is essentially a form of high negative cooperativity. Our NMR studies of ecTS (from E. coli) will also focus on this intersubunit negative cooperativity and the structural and dynamic features associated with it. Strikingly, hTS (from human) shows positive cooperativity in binding substrate, while still exhibiting half-site-reactivity. In summary, TS is a fascinating, large enzyme that is interesting from both the perspective of catalysis and allostery.
One of the most intriguing features of hTS is that it contains a flexible N-terminus (two actually, it’s a homodimer!) that imparts functional properties to the enzyme through unknown mechanisms. Projects in the lab are addressing the physical behavior of the N-terminus and what functional features of the enzyme depend on its presence.