Elucidating mechanisms of addiction
Goals: Our primary goals are to: 1) shed new light on the neural mechanisms that drive the positive reinforcing effects of alcohol and other drugs of abuse; and 2) investigate the impact of alcohol use on neurodegenerative disorders including Alzheimer’s disease.
Strategy: Our preclinical studies take a “discovery-to-mechanistic” approach: 1) we seek to discover novel neural targets of alcohol use (changes in gene and protein expression); and 2) experimentally manipulate key targets using pharmacological or genetic strategies to determine if specific targets mechanistically regulate excessive alcohol self-administration and related behavioral pathologies.
Significance: Understanding the neural mechanisms of drug-induced maladaptive plasticity in brain and behavioral functions is important for development of new pharmacotherapeutic strategies for treating problems associated with alcohol and drug abuse, and neurodegenerative disorders.
Featured research
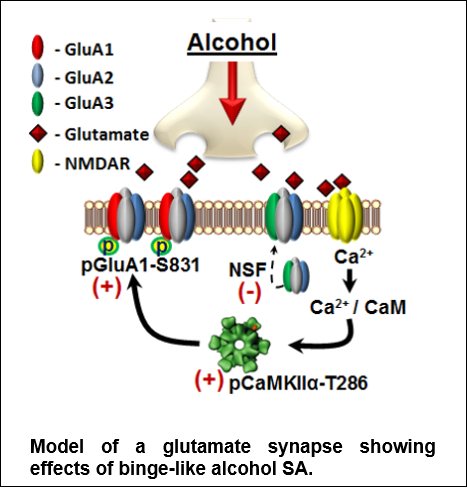
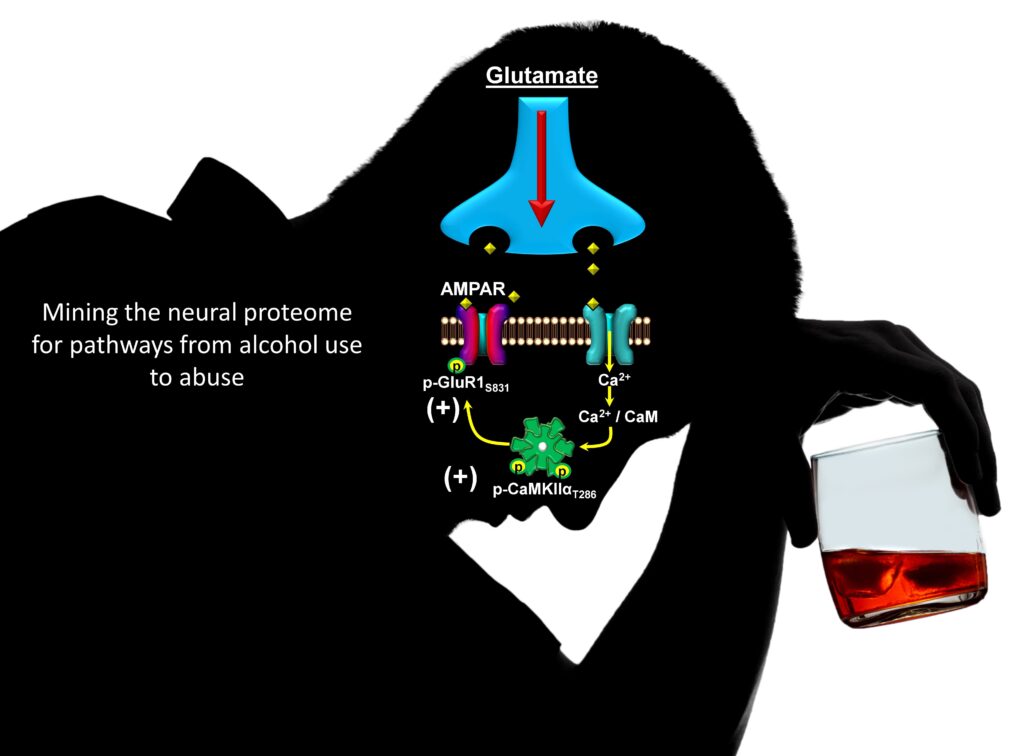
Discovery: The figure shows a model of CaMKII in glutamate synapse. We discovered that moderate (nondependent) alcohol self-administration produces major effects on the neuro-proteome, including maladaptive alterations in cell signaling proteins that controls neuroplasticity. Accordingly, our work identified CaMKII (a.k.a. the molecular memory switch) as a primary target of alcohol that, in turn, drives the positive reinforcing effects of the drug. Now, we are focused on TARP gamma-8, which is required for CaMKII modulation of AMPAR-mediated glutamate signaling. Based on Salling et al., 2016.
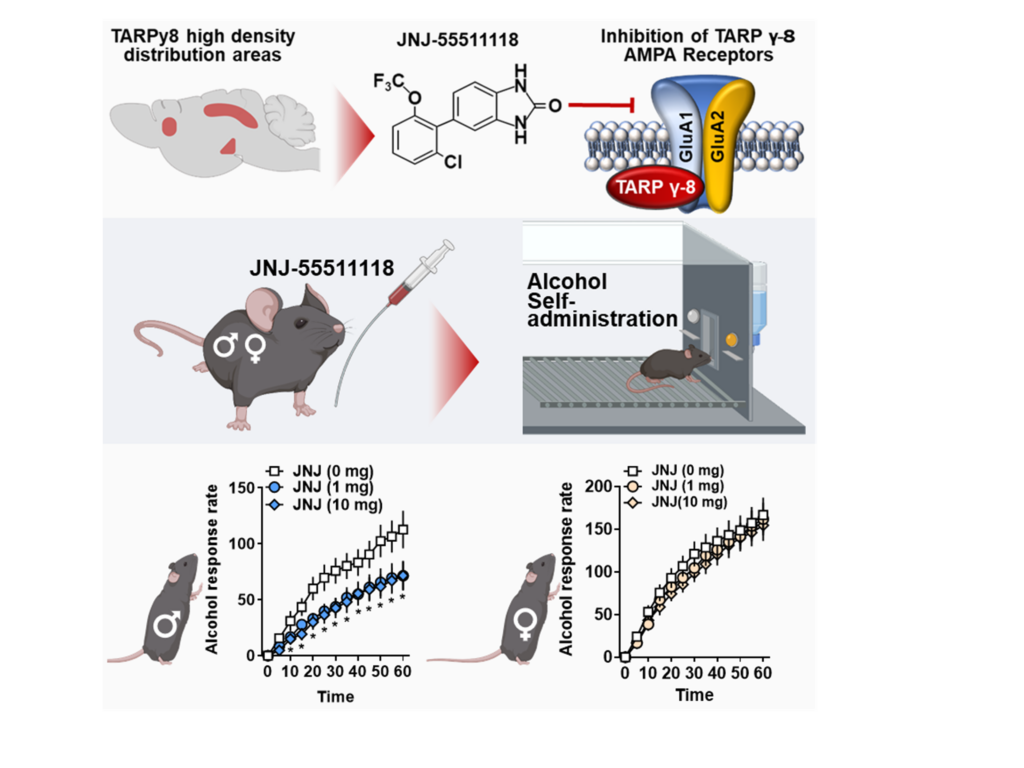
Mechanism: This figure shows a summary of our preclinical medications development work. Glutamate AMPAR function is modulated by TARP γ-8. We evaluated JNJ-55511118, a novel inhibitor of TARP γ-8 bound AMPARs, for preclinical efficacy in reducing chronic alcohol self-administration. JNJ-55511118 decreased operant alcohol self-administration in C57BL/6J mice, demonstrating TARP γ-8 bound AMPAR activity is required for alcohol reinforcement. This discovery shows for the first time that TARP γ-8 regulates behavioral pathology associated with addiction. From Hoffman et al., 2021.
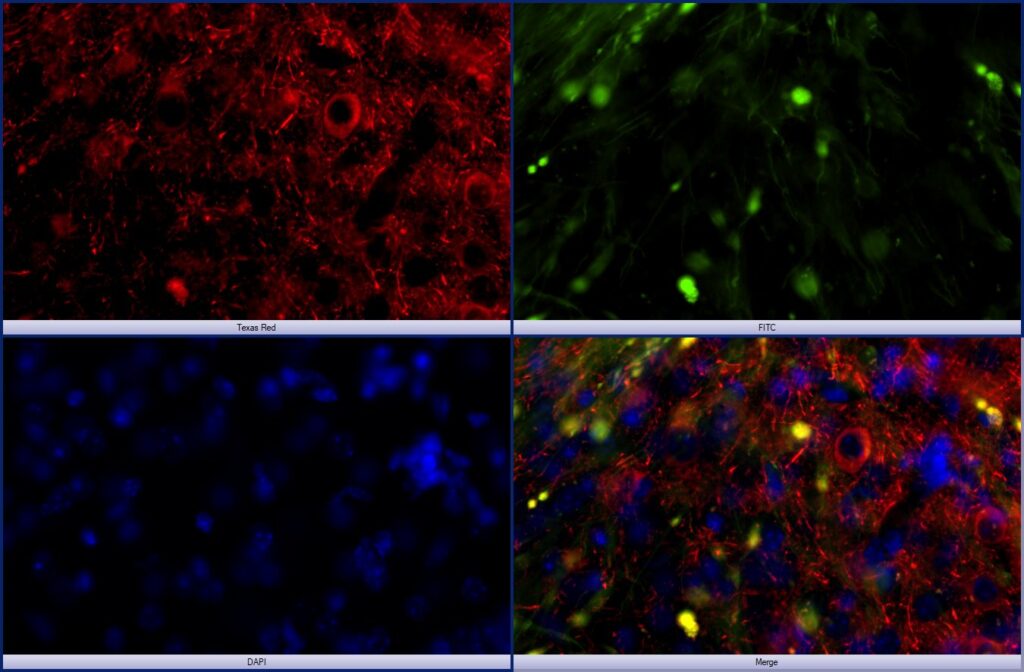
Neurodegeneration: The figure at left shows an experimental model of human Tau propagation in the mouse amygdala after “seeding” in the entorhinal cortex via an AAV approach. Human tau (red), AAV-GFP (green), DAPI (blue). In this ongoing study, we are evaluating the hypothesis that alcohol use exacerbates Tau propagation in the brain.