Research
Our research interests focus on the immunologic and genetic mechanisms of lymphomagenesis, particularly in the setting of HIV infection. While hematologic malignancies and lymphoproliferative disorders in sub-Saharan Africa (SSA) arise under intrinsic and extrinsic pressures very different from those in the United States, comprehensive analyses of these diseases have not been performed.
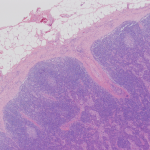
Reactive Lymph Node
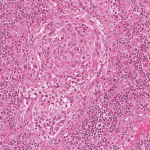
Multicentric Castleman Disease
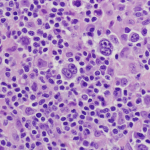
Hodgkin Lymphoma
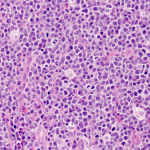
Diffuse Large B Cell Lymphoma
We use advanced sequencing, immunophenotypic and cellular analyses to address gaps in our understanding of lymphomagenesis and tumor microenvironment in the context of HIV-associated immune dysregulation, with the goal of translation to clinical care and future clinical trials.
KCH Lymphoma Study
The Kamuzu Central Hospital (KCH) Lymphoma Study began in 2013 in response to increasing lymphoma burden in Malawi and lack of pathology services. Cases in this prospective cohort, with over 500 patients enrolled in 2021, are diagnosed through weekly telepathology consultation involving multiple pathologists in Malawi and the US who render a consensus opinion.
See the Global Diagnostics page for additional information.
Lymphoma Biology
Diffuse Large B-cell Lymphoma (DLBCL), the most common lymphoma worldwide and in SSA, is highly associated with HIV, but thorough studies of HIV-associated DLBCL are globally scarce. While the striking genetic heterogeneity of sporadic DLBCL in HIV-naïve patients has been extensively studied, this work has been challenging to conduct in HIV-positive patients. Independent studies in HIV-positive patients provide unprecedented and generalizable insight into lymphoma biology and inform prevention and treatment strategies regionally and worldwide.
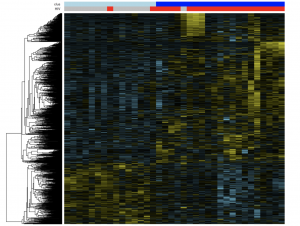
Unsupervised hierarchical clustering of RNA-seq data from Malawi DLBCL cases demonstrate a strong contribution of HIV status to DLBCL expression phenotype, with the majority of HIV-positive DLBCL cases clustering together. Mechanisms underlying this phenomenon are unclear but may reflect systemic or microenvironmental pressures on lymphoma development or evolution in the unique setting of HIV infection.

Compared to HIV-negative cases, HIV-associated DLBCL was enriched for hypoxia-induced genes and expression modules related to oxidative stress, and the expression cluster heavily enriched for HIV DLBCL also showed significant differences related to angiogenesis.
Tumor Microenvironment
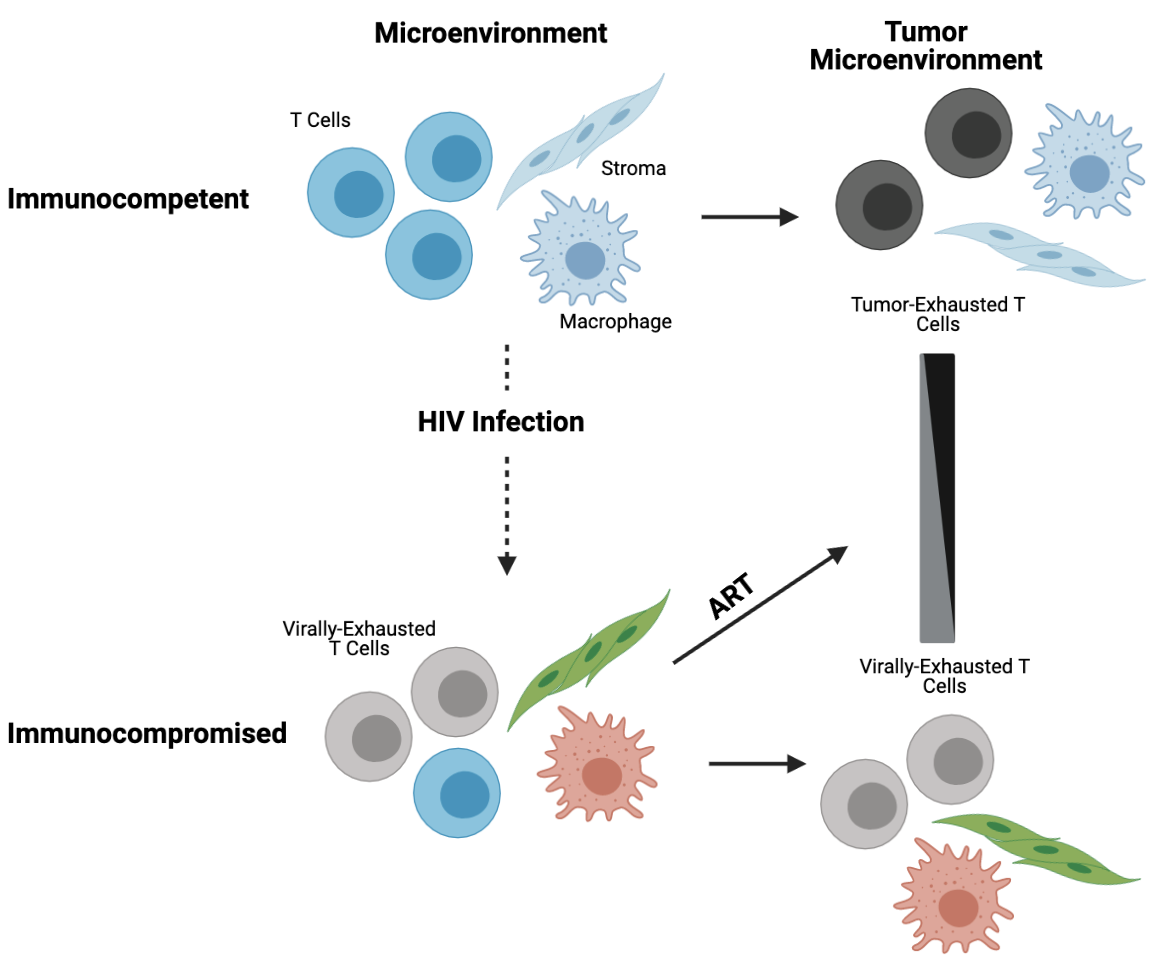
Tumorigenesis, in immunocompetent and immunodeficient hosts, is driven by somatic evolution. The rate, number, and diversity of genetic changes are impacted by selective pressure in the local microenvironment. Diffuse large B-cell lymphoma (DLBCL) is highly associated with HIV and the commonest cause of cancer death among HIV-infected individuals. HIV-associated DLBCL develops in the setting of varying degrees of HIV replication, immune dysregulation, and B-cell activation. Despite having histologically similar appearances, HIV-associated DLBCL likely differs biologically compared to DLBCL in immunocompetent populations and may also biologically differ within HIV-infected populations depending on the HIV immunologic and virologic environment in which it occurs. Current diagnostic and therapeutic strategies do not account for these differences, and a clearer understanding of selective pressures in HIV-associated DLBCL can inform treatment strategies for this highly heterogeneous cancer.
HHV8-Associated Multicentric Castleman Disease
Human Herpesvirus 8-Associated Multicentric Castleman Disease (MCD) is a polyclonal B-cell lymphoproliferative disorder that occurs almost exclusively in people living with HIV (PLWH). With the number of PLWH increasing, improvements in MCD diagnosis and risk stratification are necessary. We aim to enhance our understanding of MCD biology through RNA and T cell receptor sequencing to ultimately help predict development/progression of MCD.
Recent Publications
Roush SM, Coelho J, Xu AM, Puranam K, Mponda M, Kasonkanji E, Mulenga M, Tomoka T, Galeotti J, Brownlee A, Ghadially H, Chagomerana M, Damania B, Painschab M, Merchant A, Gopal S, Fedoriw Y. HIV infection and ART exposure affect tumor TCR repertoire of diffuse large B cell lymphoma. JCI Insight. 2024 May 23;9(13):e180771. doi: 10.1172/jci.insight.180771. PMID: 38781015; PMCID: PMC11383373.
, , , , , , et al. HIV and prior exposure to antiretroviral therapy alter tumour composition and tumour: T-cell associations in diffuse large B-cell lymphoma. Br J Haematol. 2024; 205(1): 194–206. https://doi.org/10.1111/bjh.19531
Brownlee AJ, Dewey M, Chagomerana MB, Tomoka T, Mulenga M, Khan S, Kampani C, Chimzimu F, Gastier-Foster JM, Westmoreland KD, Ozuah NW, Krysiak R, Malamba-Banda C, Painschab MS, Gopal S, Fedoriw Y. Update on pathology laboratory development and research in advancing regional cancer care in Malawi. Front Med (Lausanne). 2024 Jan 16;11:1336861. doi: 10.3389/fmed.2024.1336861. PMID: 38298817; PMCID: PMC10829605.
Razzano D, Puranam K, Tomoka T and Fedoriw Y (2022) The role of telepathology in improving cancer diagnostic and research capacity in sub-Saharan Africa. Front. Med. 9:978245. doi: 10.3389/fmed.2022.978245
Kimani SM, Painschab MS, Horner MJ, Muchengeti M, Fedoriw Y, Shiels MS, Gopal S. Epidemiology of haematological malignancies in people living with HIV. Lancet HIV. 2020 Sep;7(9):e641-e651. doi: 10.1016/S2352-3018(20)30118-1. Epub 2020 Aug 10. PMID: 32791045.
Fedoriw Y, Selitsky S, Montgomery ND, Kendall SM, Richards KL, Du W, Tomoka T, Mulenga M, Parker JS, Dave SS, Gopal S. Identifying transcriptional profiles and evaluating prognostic biomarkers of HIV-associated diffuse large B-cell lymphoma from Malawi. Mod Pathol. 2020 Aug;33(8):1482-1491. doi: 10.1038/s41379-020-0506-3. Epub 2020 Feb 20. PMID: 32080349.
Lilly AJ, Fedoriw Y. Human Immunodeficiency Virus-Associated Lymphoproliferative Disorders. Surg Pathol Clin. 2019 Sep;12(3):771-782. doi: 10.1016/j.path.2019.03.005. Epub 2019 May 17. PMID: 31352987.
Painschab MS, Kasonkanji E, Zuze T, Kaimila B, Tomoka T, Nyasosela R, Nyirenda R, Dhungel BM, Mulenga M, Chikasema M, Tewete B, Mtangwanika A, Chiyoyola S, Mhango W, Chimzimu F, Kampani C, Krysiak R, Shea TC, Montgomery ND, Fedoriw Y, Gopal S. Mature outcomes and prognostic indices in diffuse large B-cell lymphoma in Malawi: a prospective cohort. Br J Haematol. 2019 Feb;184(3):364-372. doi: 10.1111/bjh.15625. Epub 2018 Nov 18. PMID: 30450671; PMCID: PMC6340743.
Tomoka T, Montgomery ND, Powers E, Dhungel BM, Morgan EA, Mulenga M, Gopal S, Fedoriw Y. Lymphoma and Pathology in Sub-Saharan Africa: Current Approaches and Future Directions. Clin Lab Med. 2018 Mar;38(1):91-100. doi: 10.1016/j.cll.2017.10.007. Epub 2017 Dec 13. PMID: 29412887; PMCID: PMC5999328.
Montgomery ND, Tomoka T, Krysiak R, Powers E, Mulenga M, Kampani C, Chimzimu F, Owino MK, Dhungel BM, Gopal S, Fedoriw Y. Practical Successes in Telepathology Experiences in Africa. Clin Lab Med. 2018 Mar;38(1):141-150. doi: 10.1016/j.cll.2017.10.011. Epub 2017 Dec 6. PMID: 29412878; PMCID: PMC5996143.
Funding
HIV-Associated DLBCL as a Spontaneous Model System to Investigate Tumor-T cell Interactions (UNC LCCC Developmental Funding Award)
Malawi Cancer Outcomes Research Program (M-CORP, NIH D43)
UNC-Malawi-South Africa Cancer Consortium (UMSACC, NIH U54)
Integrated Translational Oncology Program (iTOP, T32 Training Grant)