Enteroendocrine regulation of enterocyte function. We previously found that EECs influenced ion transport and therefore glucose and peptide absorption in enterocytes (McCauley et al, Nat Comms 2020). Ion transport and nutrient availability are linked to cellular metabolism and mitochondrial function, which afford enterocytes the capacity to absorb sufficient nutrients to survive. The role of EECs in regulating these processes has been difficult to study due to absence of good model systems – until now. This project employs metabolomics, gene expression analysis, and functional assays in both mouse models and PSC-derived human intestinal organoids to identify the roles for individual EEC-derived hormones in regulating their enterocyte neighbors. Funded by a K01 Career Development Award from NIDDK, 2021-2026.
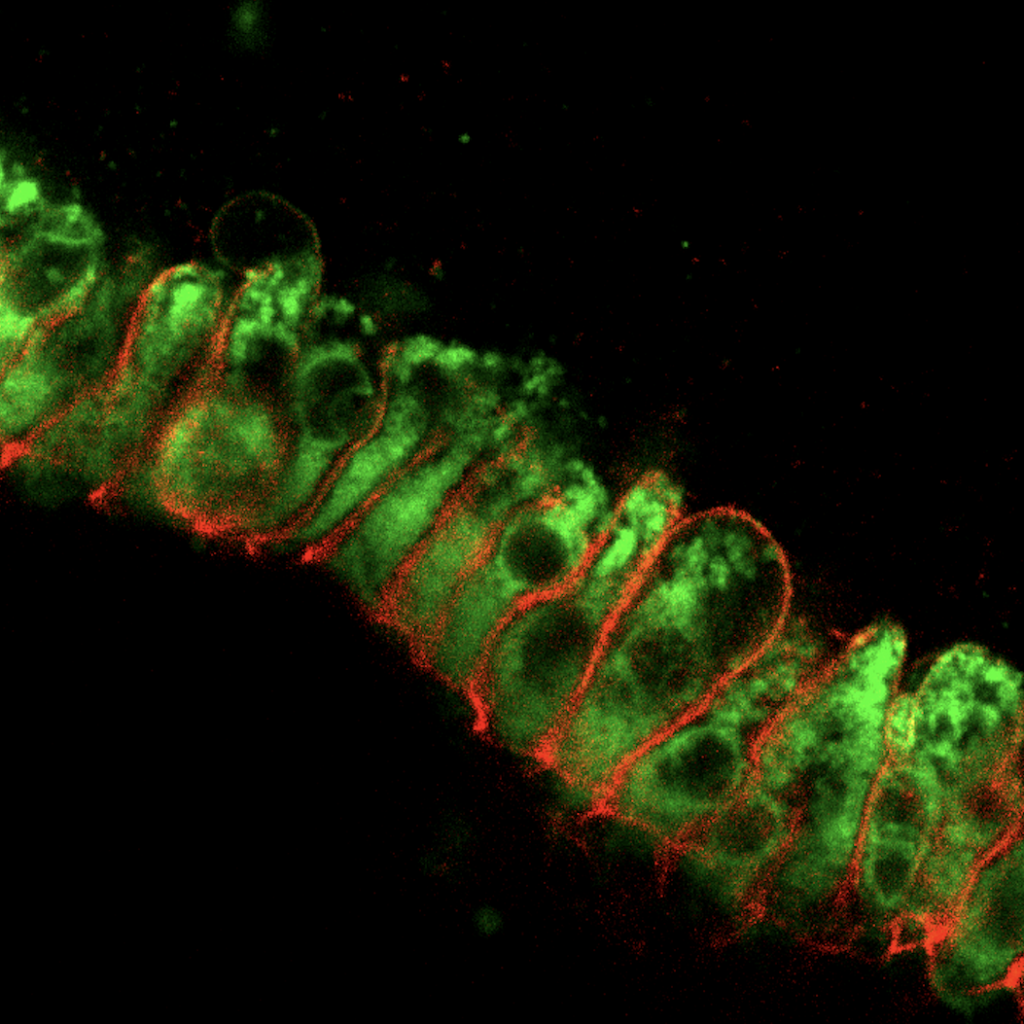

EEC regulation of barrier function. EECs are often dysregulated in inflammatory bowel disease (IBD), characterized by mucosal inflammation and impaired barrier function. We hypothesize that EECs strengthen the epithelial barrier by regulating structural proteins and lipids, and that EECs may be targeted to improve symptoms of IBD. This project combines tissue culture work using epithelial monolayers derived from HIOs and a mouse model of colitis.
EEC regulation of crypt metabolism. We recently found that EECs are required to restrict intestinal crypt cells, including stem, progenitor, and Paneth cells, from adopting a fatty acid metabolic program (McCauley et al, CMGH 2023). This project delves deeper into metabolic profiling of crypts with and without EECs and establish roles for individual EEC peptides or metabolites in the regulation of crypt metabolism and homeostasis, in both mouse and in human PSC-derived human intestinal organoids. Funded by a K01 Career Development Award from NIDDK, 2021-2026.
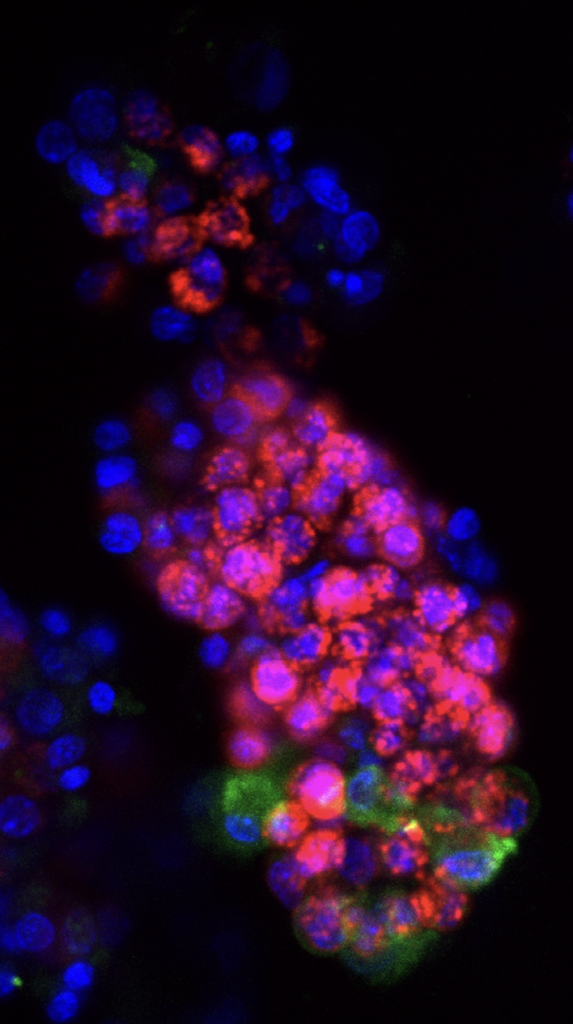
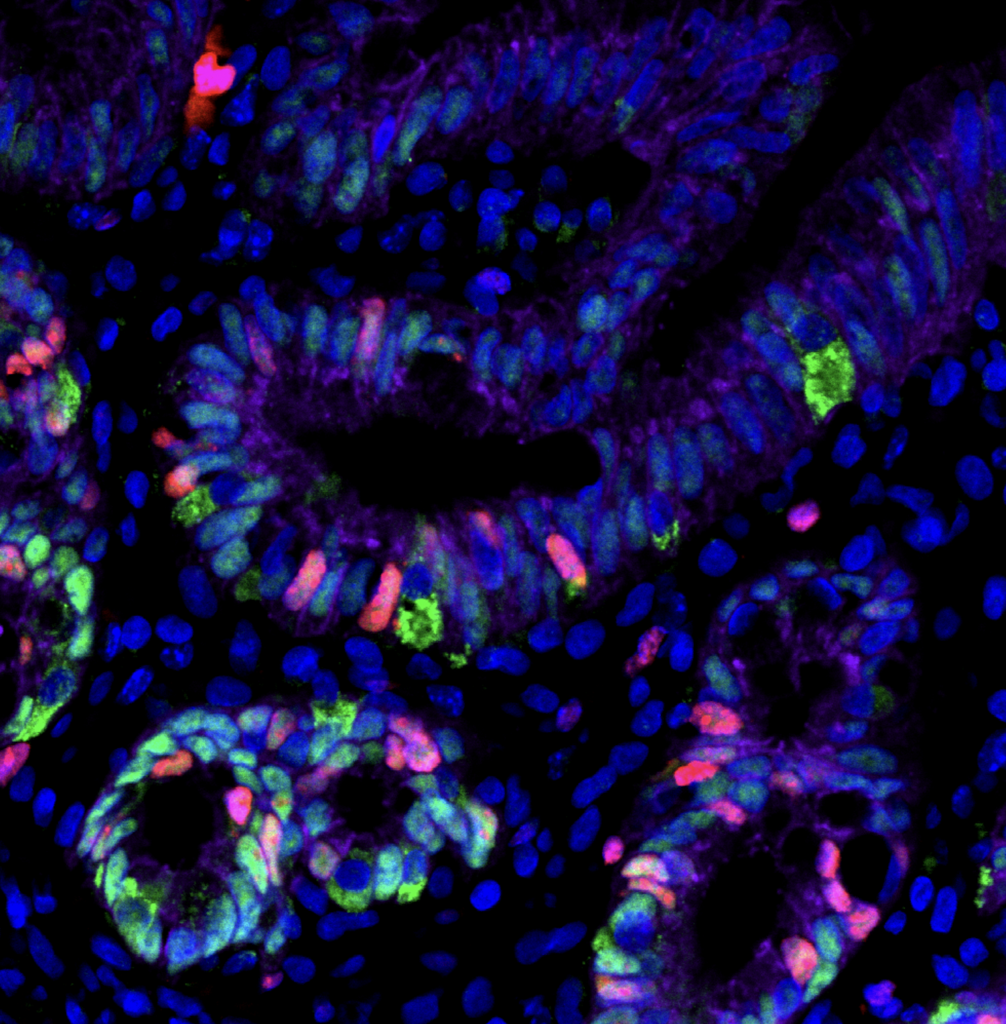
Enteroendocrine regulation of signaling pathways governing homeostasis. The balance between proliferation and differentiation in the intestine is essential for homeostasis. We recently demonstrated that EECs are essential regulators of cellular metabolism in the crypt, and that in their absence, intestinal stem cell activity and cellular proliferation is increased (McCauley et al, CMGH 2023). This led us to hypothesize that enteroendocrine cells may influence the master signaling pathways Wnt and BMP which regulate cellular proliferation and differentiation. Because increased proliferation and imbalance in the signaling pathways that maintain intestinal homeostasis could potentially drive tumor formation, we will investigate the role of EECs in a mouse model of colorectal cancer driven by an activating mutation in the Wnt pathway. Funded by a Pilot & Feasibility award from the Center for Gastrointestinal Biology and Disease, 2023-2024.
EECs may act as tumor suppressors. The mechanisms connecting lifestyle factors, such as diet, with increased risk for colorectal cancer are unknown. Here, we hypothesize that the nutrient-sensing EEC protects the intestinal epithelium from oxidative stress and activates DNA damage repair pathways that prevent the accumulation of oncogenic mutations in proliferative cells. EEC function is impaired by diets high in fat, and high-fat diet promotes intestinal tumorigenesis in animal models. In the context of APC mutation, a common mouse model for colorectal cancer, we found that loss of EECs increases tumor burden throughout the gastrointestinal tract. Funded by a Developmental Award by the Lineberger Comprehensive Cancer Center, 2024-2025.
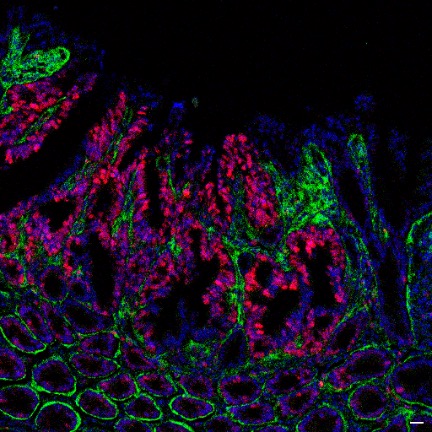
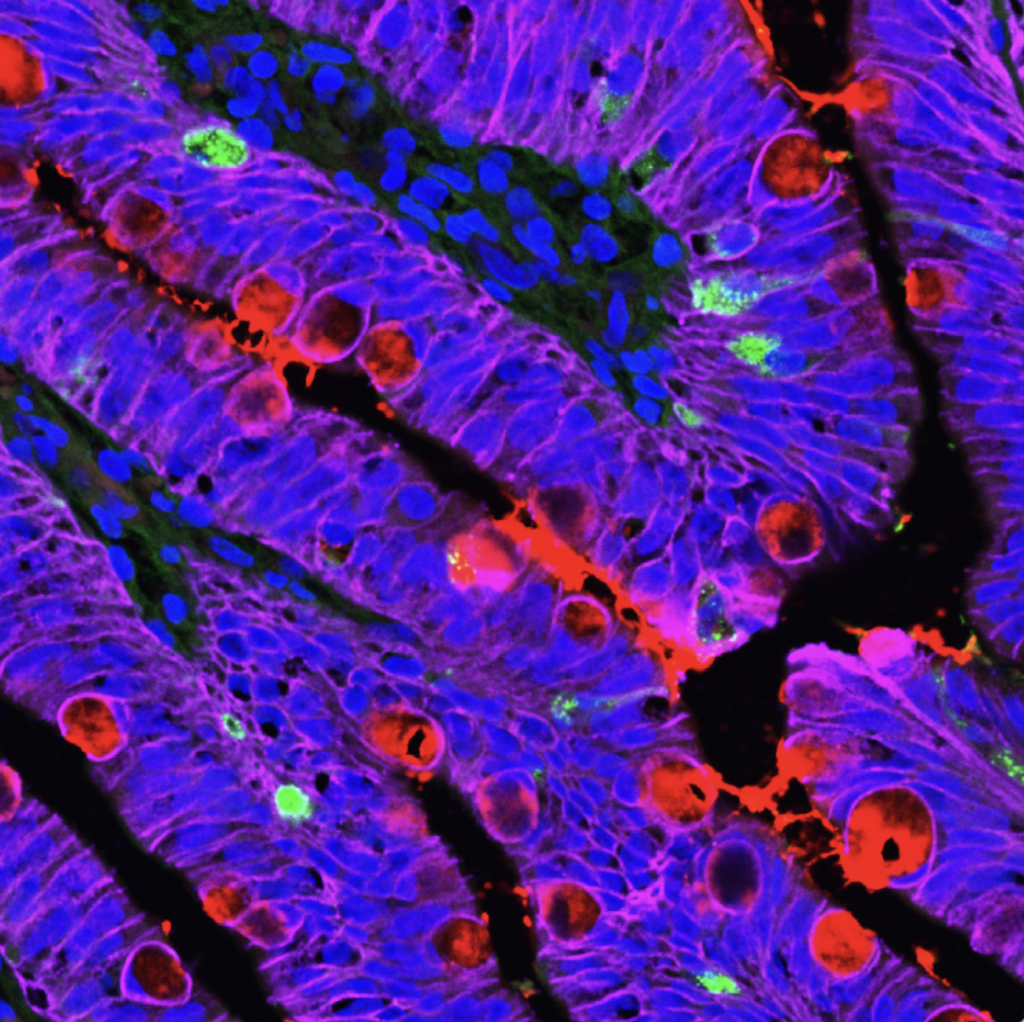
GI Manifestations of Cystic Fibrosis. GI dysfunction is common in people with CF, including nutrient malabsorption, increased risk for cancer, and intestinal obstructions. These issues are relatively understudied compared to lung disease in people with CF. It has been difficult to resolve the relative contributions of abnormal mucus, altered microbiome, immune activation, and epithelial-specific disruptions to these processes. We use HIOs to test the hypothesis that nutrient absorption in people with CF is impaired by altered mucus composition and aberrant intestinal cell biology. Future goals include evaluating the contribution of EECs to CFTR function and pathophysiology in people with CF. Funded by the Cystic Fibrosis Foundation, 2024-2026.
EEC regulation of enteric neuron development. The enteric nervous system (ENS) innervates the intestine, controlling important processes like gut motility and water resorption. The gut is also innervated by vagal afferents which signal directly to the brain. EECs and enteric and vagal neurons form synaptic connections that allow for rapid communication between these essential cell types in the intestine. In addition to producing hormones, EECs also produce neurotrophic factors, raising the possibility that EECs help attract neurons to the right place in the developing intestinal epithelium and instruct them to become the right type of neuron.
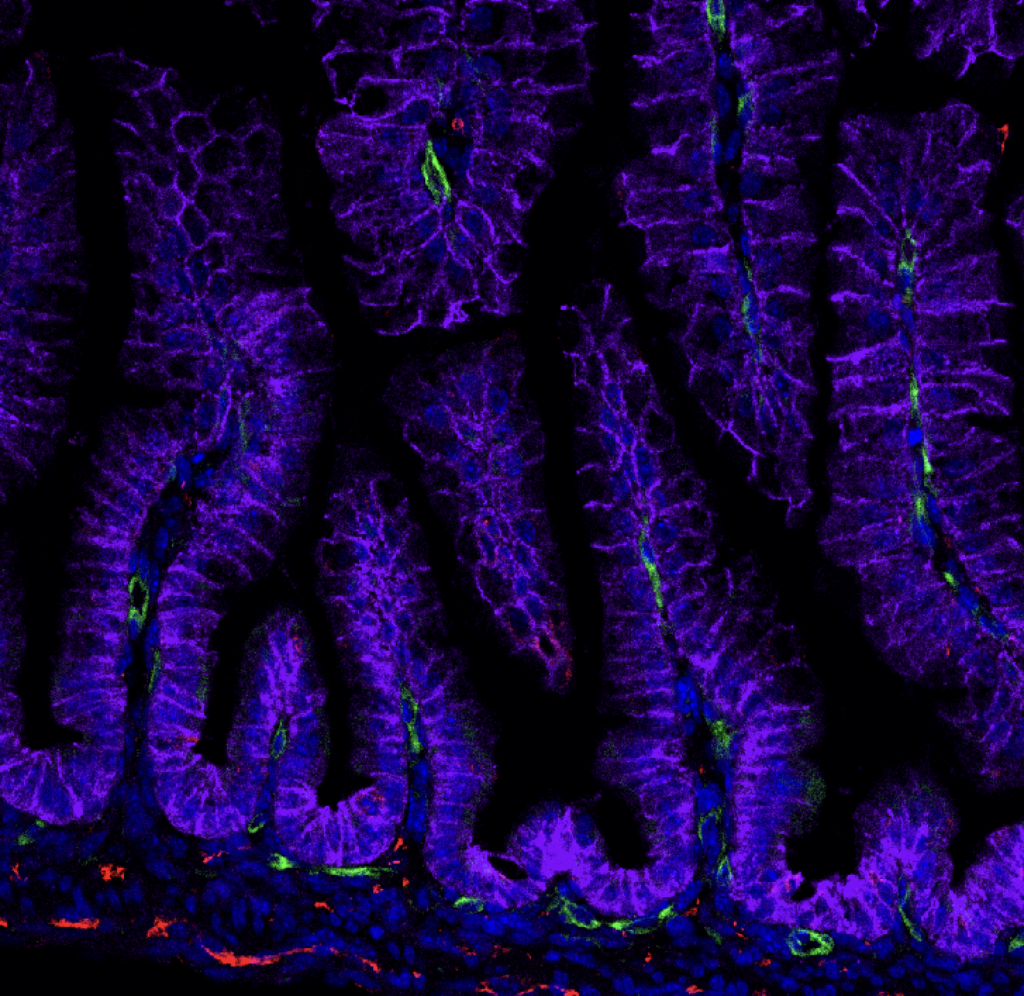
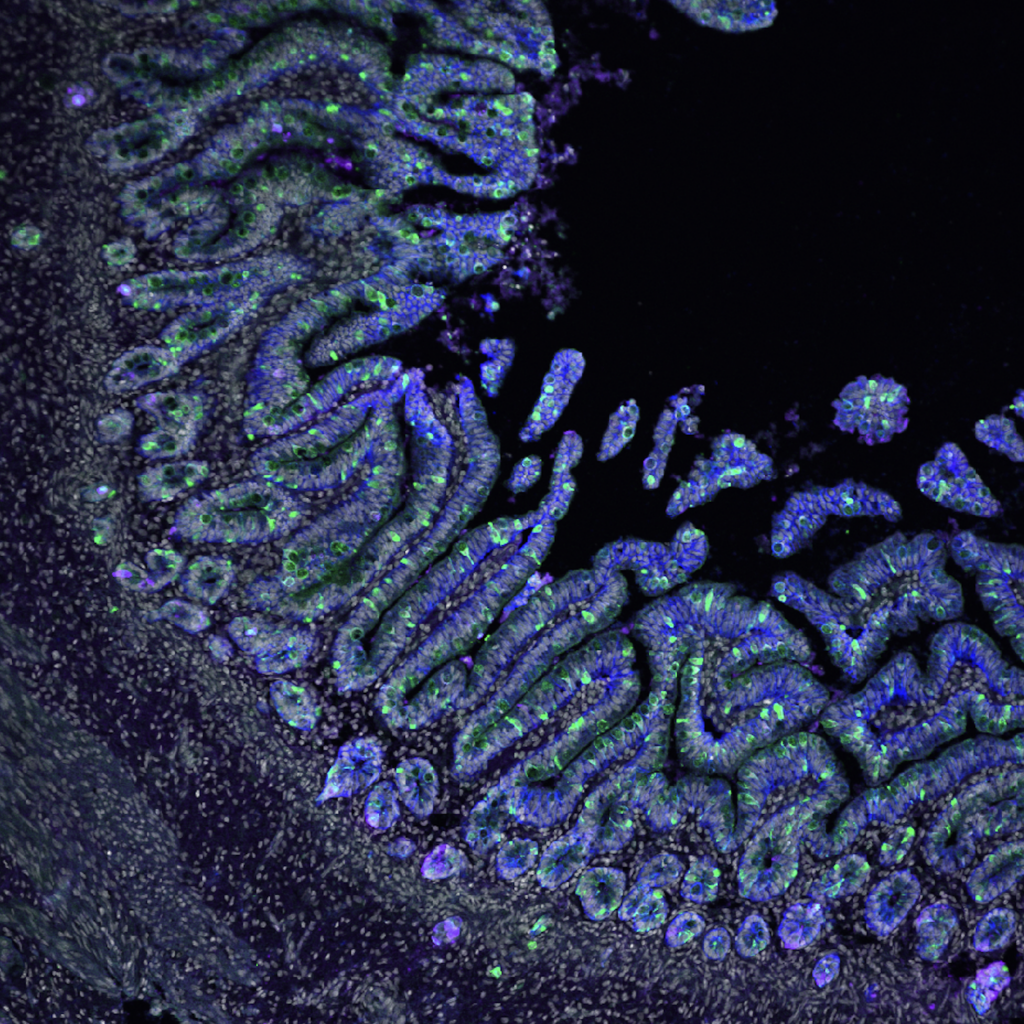
Open to your ideas! The McCauley Lab believes in intellectual equity, meaning the best ideas can come from anyone. Pitch your project and we’ll see if we can get it off the ground using the tools we have!