Everything was perfect. I was building the surveys, practicing the lattes, and preparing for clinical days. Then the house of cards that was my summer felt a light gust and fell apart. That is a wildly exaggerated metaphor but keep an open mind. I spent much of my summer dividing my time between the three jobs of being a barista, a student, and a biostatistics intern. Three jobs may sound like too much work… and it was.
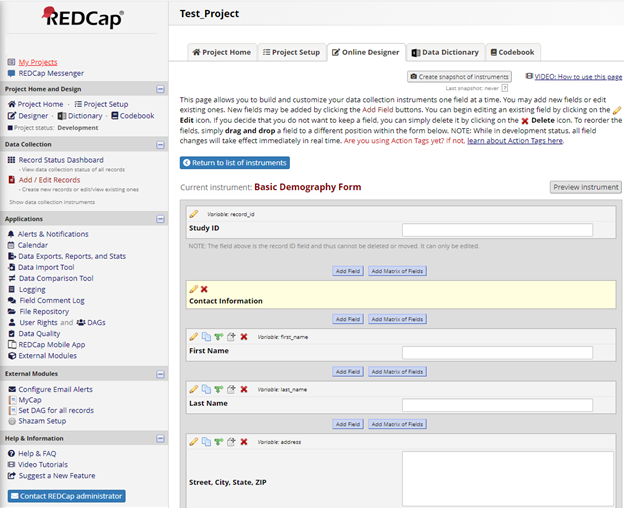
Over the past two months I have been working with Dr. Jessica Keys to prepare a database for a clinical trial with the UNC Center For Aids Research. Much thought went into the structure of the electronic case report forms or eCRFs that we were creating. Aside from coding and translating forms, most of the meetings with my preceptor involved brainstorming rather than hands-on work to discuss the sequence in which the forms would be filled out and how that would influence the coding within each form. For example, many of the forms had redundant information such as date of birth or height. So, we used coding to fill in the redundant information automatically by transferring the data from one form to another. Overall, the practicum gave me my first experience with creating a database and my first practical experience in biostatistics. My preceptor stressed all the best practices that one can do to make the data analyst’s job more efficient. For instance, one should export the data early in the process to see how it looks and adjust the forms if needed. Other tips included keeping the number of variables small if possible and create self-explanatory variables to make the data analysis more user-friendly. More important than the lessons I learned, I am now able to use REDCap and plan to learn more biostatistics skills to explore other positions in the future.
What did I do when I wasn’t creating digital surveys? I finished training as a barista in March and will continue to work 20 hours a week indefinitely. Creating lattes is a nice break from academics and I enjoy learning about all the special types of coffee that exist. It’s crazy how many ways there are to make coffee. And something I have learned this summer is that it is essential to have something built into your schedule that allows you to relax.

(https://cdn.shopify.com/s/files/1/1379/8821/files/cafes-drmwood_0000s_0001_Layer_22_6167444c-37a4-4027-8e36-b289fed3227e.jpg?v=1644962612)
Finally, I took an EMT course. The lectures were 16 hours a week at night and all-day Saturday, but I was glad to be busy this summer. Unfortunately, I failed one of the last exams in the course and had to stop two weeks before completing the class! But on the other hand, I worked one emergency department shift before failing and had the chance to practice a lot of what I learned during the semester.
I admit that I was greedy. I tried to work 40 hours a week while taking night classes rather than prioritizing the EMT course, but I still debate whether I should commit to studying or continue to serve coffee to relax. For now, I will be on the streets of Durham if anyone needs CPR, a latte, or a database.